Standard Gibbs Free Energy Formula
G H - TS If the reaction is run at constant temperature this equation can be written as follows. ΔG ƒ ΔH ƒ compound - TΔS compound Values of standard Gibbs free energy of formation are tabulated for many compounds.

Temperature In The Gibbs Free Energy Equation Chemistry Stack Exchange
Also calculates the change in entropy using table of standard entropies.
Standard gibbs free energy formula. So if you had to calculate the Gibbs free energy change at say 298 K you can just slot the numbers in. Gibbs free energy equation. Gibbs Free Energy Equation Gibbs free energy is equal to the enthalpy of the system minus the product of the temperature and entropy.
The equation is given as. ΔH change in enthalpy. When a system changes from an initial state to a final state the Gibbs free energy ΔG equals the work exchanged by the system with its surroundings minus the work of the pressure force.
And youll get minus 890 point 3 kilojoules whats tells us that this is an exothermic reaction that this side of. ΔH -8904 kJ mol-1. We can use the relationship between ΔG and the equilibrium constant K to obtain a relationship between E cell and K.
The following equation relates the standard-state free energy of reaction with the free energy at any point in a given reaction not necessarily at standard-state conditions. Determining if a reaction is spontaneous by calculating the change in Gibbs free energy. Gibbs Free energy formula is given below.
Recall that for a general reaction of the type aA bB cC dD the standard free-energy change and the equilibrium constant are related by the following equation. 129 rows The standard Gibbs free energy of formation Gf of a compound is the change of Gibbs. K eq the ratio C D A B at equilibrium is called the equilibrium constant.
The change in the Gibbs free energy of the system that occurs during a reaction is therefore equal to the change in the enthalpy of the system minus the change in the product of the temperature times the entropy of the system. G H - TS. Standard change in free energy and the equilibrium constant.
G H TS. Delta G Delta Go RT ln Q label110 Delta G free energy at any moment Delta Go standard-state free energy. ΔG ΔH - TΔS ΔG -8904 - 298-02442 -8176 kJ mol-1.
Standard Gibbs free energy of formation of a compound can be calculated using standard enthalpy of formation ΔH ƒ absolute standard entropy ΔS and standard temperature T 29815 K. Solving for G 0 yields the relationship at left. The Gibbs free energy equation is dependent on pressure.
You must convert your standard free energy value into. Where G change in free energy G 0 standard free energy change with 1 M reactants and products at pH 7 R gas constant T absolute temperature At equilibrium G equals zero. 2 moles -2372 kjmole -4744 kj Standard Free Energy of Formation for two moles H2O l Look for the Standard Free Energy of Formation of CO2g and multiply by its coefficient 4.
The maximum work done is the amount of energy produced given by the decrease in the thermodynamic property called Gibbs free energy. The units of ΔG If you look up or calculate the value of the standard free energy of a reaction you will end up with units of kJ mol-1 but if you look at the units on the right-hand side of the equation they include J - NOT kJ. 378 rows See also Standard enthalpy of formation Gibbs free energy of formation entropy and.
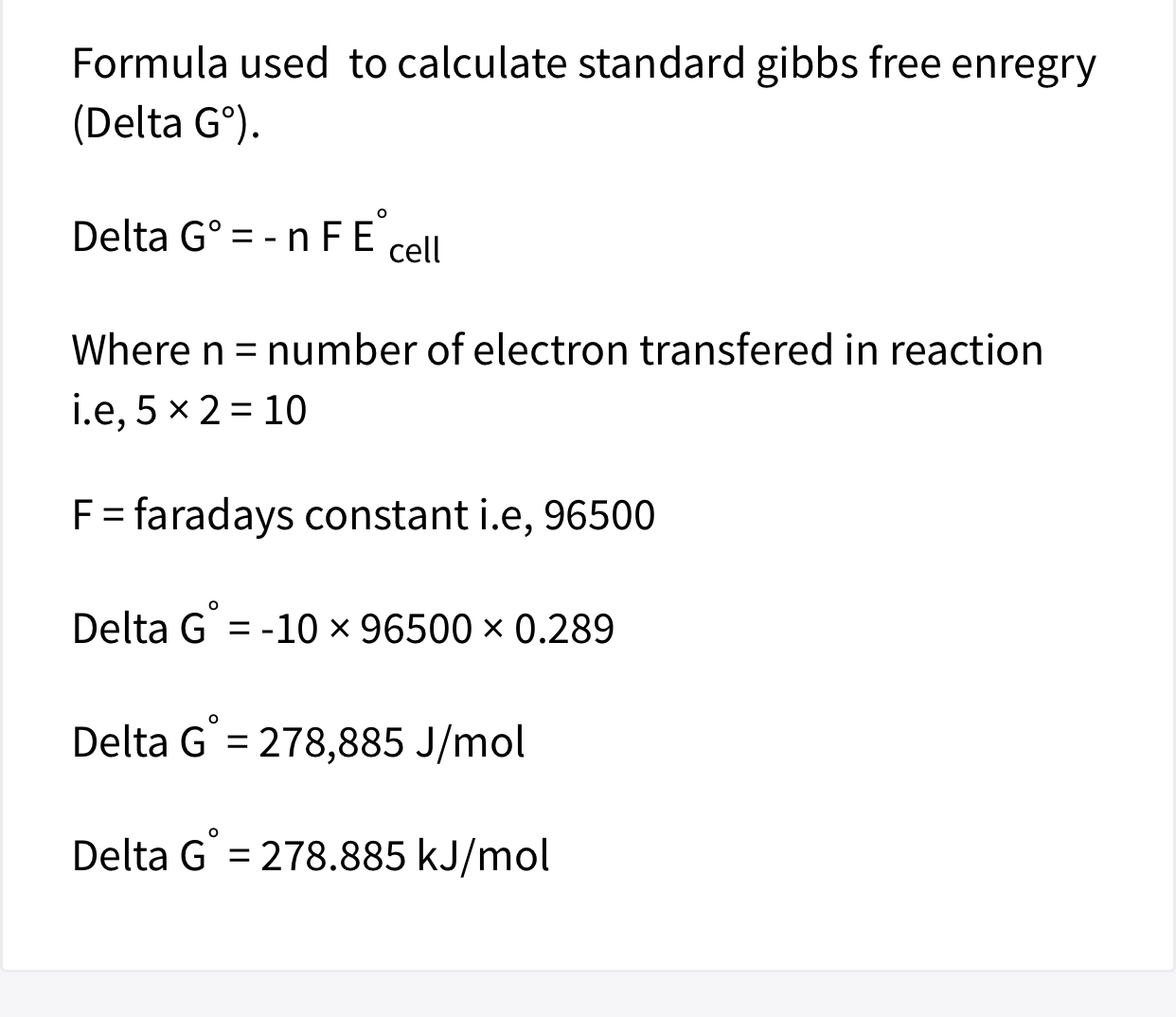
Look up the Standard Free Energy of Formation of H2O g and multiply by its coefficient 2 in the equation. 723 Gibbs free energy The standard electrode potential is related to changes in Gibbs free energy by the following equation 79 Δ G 0 n F E 0 where ΔG0 is the change in standard Gibbs free energy of the reaction and n is the number of electrons participating in the reduction reaction.
